

The Power of Data
CHAMPS’ precise, accurate, real-time data explains why children under five are dying.
- SEE ALSO DATA ON:
- Neonate
- Infant & Child
- Populations Enrolle
- Test
Explore the Data
Four Levels of Data Access
These data provide summaries of the CHAMPS-eligible and enrolled cases at each surveillance site and determined causes of death across each of our sites. Causes of death can be filtered by CHAMPS site, etiology, CHAMPS mortality categories, age group, and other categories. Causes of death can also be viewed by all causes and specific etiology.
Data summaries are updated daily.
- Level 1: Summarized Data
- Level 2: De-Identified Data
- Level 3: Limited Data
- Level 4: Potentially Identifiable Data
Summarized data are updated in real-time and available to all on our web site with one click. These data provide summaries of the CHAMPS eligible and enrolled cases at each surveillance site, as well as determined causes of death across each of our sites. Cause of death data are categorized by Stillbirth, Neonate, and Infant and Child, and provide separate views for all causes of death, and infectious etiologies. Data summaries are updated daily.
De-identified data can be downloaded as a curated standard data set after registration and electronic acceptance of a Data Transfer Agreement (DTA).
Standard data set includes:
- Case demographics (including MITS measurements), verbal autopsy, DeCoDe results, TAC results, and lab results
- Additional curated standard datasets in development
- Data transformations performed to de-identify this dataset: Case identifiers replaced with surrogates; dates shifted; narrative/summary fields removed
To view documentation for the de-identified dataset prior to downloading, obtain descriptive metadata or the citation for the dataset, please visit the CHAMPS Dataverse and scroll to the bottom of the page.
To access the CHAMPS R package, visit our GitHub Portal. The package provides utilities to read and transform L2 s data into convenient formats, functions to compute several statistics of interest, and some utilities for presenting these statistics in various formats such as plots and HTML tables.
A curated limited data set is available following registration and execution of a Data Use Agreement (DUA). The delivery time for this data set is dependent on DUA execution, and is estimated at 4-8 weeks. Please request this data set via the link below and follow the instructions to complete and upload the Data Use Agreement (DUA).
Standard limited dataset includes:
- Case demographics (including MITS measurements), verbal autopsy, DeCoDe results, TAC results, and lab results
- Additional curated limited datasets in development
- Data transformations performed to created this limited dataset: Case identifiers replaced with surrogates; narrative/summary fields removed
To view documentation for the limited dataset prior to downloading, obtain descriptive metadata or the citation, please visit the CHAMPS Dataverse and scroll to the bottom of the page.
To access the CHAMPS R package, visit our GitHub Portal. The package provides utilities to read and transform L2 data into convenient formats, functions to compute several statistics of interest, and some utilities for presenting these statistics in various formats such as plots and HTML tables.
Potentially identifiable datasets are only available via request. A CHAMPS staff member will contact you and provide further instructions once we receive your request.
A potentially identifying data set is available following registration, execution of a Data Use Agreement (DUA), and IRB approval. Timing is dependent on IRB approval and DUA execution, and is estimated at 3-6 months.
Privacy Constraints: Dependent on IRB approval – may include case photos, geo-location coordinates, free-text and narrative fields.
Understanding the Data
Mortality Coding Background
Members of Determination of Cause of Death, or DeCoDe, panels at each CHAMPS site review all data available for each death and follow WHO guidance to attribute causes of death. These procedures include using ICD-10 (10th International Statistical Classification of Diseases and Related Health Problems) to classify conditions and recording causes of death in a format matching WHO’s death certificate. In some situations, a death will have an immediate cause (lower respiratory infection, for example) that occurred in an otherwise healthy child. In these situations, the immediate and underlying cause of death will be the same. In other situations, a death may have an immediate cause (lower respiratory infection, for example) that occurred as a consequence of a child having other diseases (HIV infection, for example). In this case, the condition that originally existed that gave rise to one or more complications that led to death is known as the underlying cause of death. Comorbid conditions are those that may have contributed to the cause of death, but not directly. CHAMPS records immediate and underlying causes of death and comorbid conditions. In rare cases, the data available to DeCoDe panels are not adequate to determine a cause of death with any certainty. In these situations, the cause of death is recorded as undetermined.
For stillbirths and newborns, causes of death are often related to conditions affecting the mother or complications of childbirth or preterm birth. For these situations, CHAMPS uses a WHO application of ICD-10 for the perinatal period, ICD-PM. Because deaths in young infants may be attributed to both perinatal causes and conditions that arise after birth, CHAMPS is working to standardize procedures for deaths occurring in this age group. In situations where ICD-10 and ICD-PM codes are inconsistent, ICD-PM principles will be applied. Note that results may change as data are updated and coding procedures become more refined.
Data Collection Methods
If a case meets CHAMPS eligibility criteria, the team seeks informed consent from the parents or guardians to further investigate the cause of death through the postmortem minimally invasive tissue sampling (MITS) procedure and laboratory investigations. The laboratory investigations include microbiology, HIV, tuberculosis and malaria testing. Additional advanced diagnostics include multiplex molecular testing for many specific viral, bacterial, fungal and parasitic pathogens as well as tissue histopathology. The site teams also request consent to perform caregiver interviews, known as verbal autopsies, to explore the symptoms and conditions that may have led to the child’s death, and to collect any clinical records. Families are free to decide whether to take part in the procedures.
For those cases in which families grant consent and the MITS procedure has been performed, completing the tests and assembling the information requires 4 months. Then, an expert panel reviews all laboratory, clinical and verbal autopsy information on each case and assigns a final cause of death. This final cause of death is then provided to the family members.
Things to Note
Only a fraction of child deaths occurring in CHAMPS sites are reflected; the numbers are not adjusted for deaths in children not enrolled or for population size. No conclusions should be made about the overall contribution of each cause to total deaths for a CHAMPS site or other population.
Data that contribute to CHAMPS cause of death determinations are complex, and we're learning how expert panels should interpret it to assign causes of death. How we report the chain of causation will likely change over time.
Results presented here will change as the CHAMPS team refines methods and cleans data. If you find information that appears incorrect or confusing, contact us.
News & Resources
CHAMPS Data in Use

Case Studies
September 6, 2023
Case Study: Creating a community-based program to transport high-risk and severely ill patients to health facilities in Manhiça district, Mozambique
The CHAMPS team in Mozambique has published a new case study on how to approach transportation issues for high-risk and severely ill patients. Data from 2015 estimates that 66.7% of the population of Mozambique were living more than 60 minutes away from a healthcare center, either by vehicle or on foot (8), and different communities (…)

Case Studies
September 6, 2023
Case Study: Health Demographic Surveillance System Mapping Experience and Mapping in Manyatta, Kenya
The CHAMPS team in Kenya has published a new case study on the integrated Health and Demographic Surveillance system (HDSS) in Manyatta, Kenya, an informal settlement area within Kisumu County, Western Kenya. This project was a collaboration between the Kenya Medical Research Institute (KEMRI), the CDC, and the Kenya Ministry of Health. The platform collects (…)
Case Examples
September 5, 2023
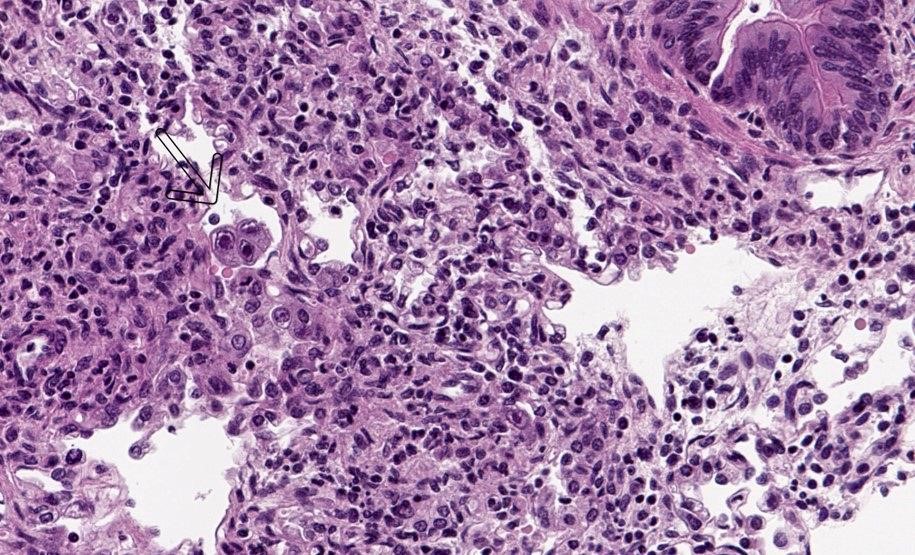
A stillbirth caused by Cytomegalovirus infection
A baby boy was stillborn at the 39th week of gestation, weighing 2.44 kg. His mother, a 19-year-old married woman, had no notable complications throughout her pregnancy, and she took iron, calcium, and folic acid during pregnancy for about a month. She reported taking some herbal medicines before her pregnancy to increase fertility. She had (…)
Case Examples
September 5, 2023
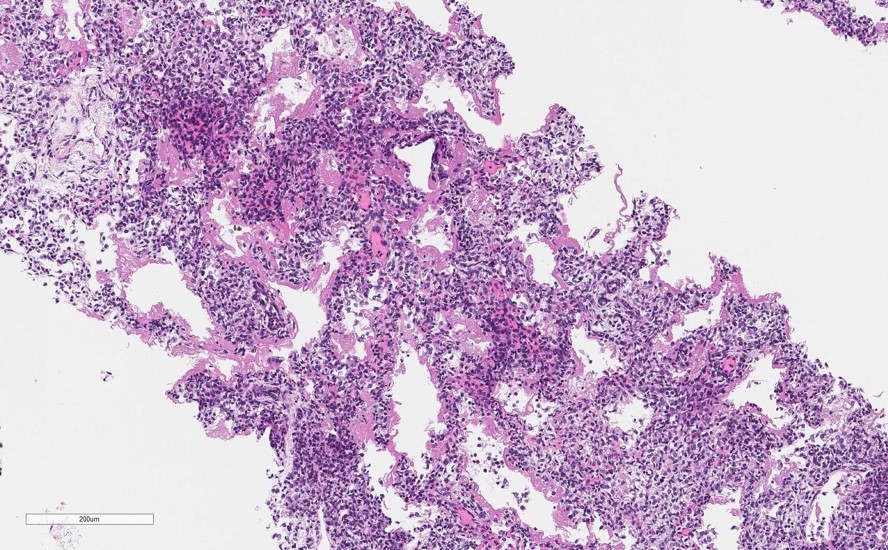
Neonatal death caused by complications of prematurity linked to maternal cervical insufficiency; possible role of anticardiolipin antibodies
A baby girl was born prematurely at about 25 weeks of gestation and died one day and 11 hours after her birth. Although the baby was born with an APGAR score of 9 and was crying, showing spontaneous movement, and had a regular heartbeat, she had difficulty breathing and could not be fed. She was (…)
News
November 8, 2022
Webinar: Addressing the Burden of Neural Tube Defects in Ethiopia
Following their presentations at this year’s ASTMH Annual Meeting, Lola Madrid and Markus Breines from the CHAMPS Ethiopia site continued the conversation on neural tube defects in Ethiopia. Lola and Markus shared highlights from the data that revealed these trends, the social-behavioral sciences approach to supporting afflicted communities, and proposed solutions to transform these findings (…)